Draw an SN1 mechanism as an example. What happens to stereochemistry in SN1 reactions.
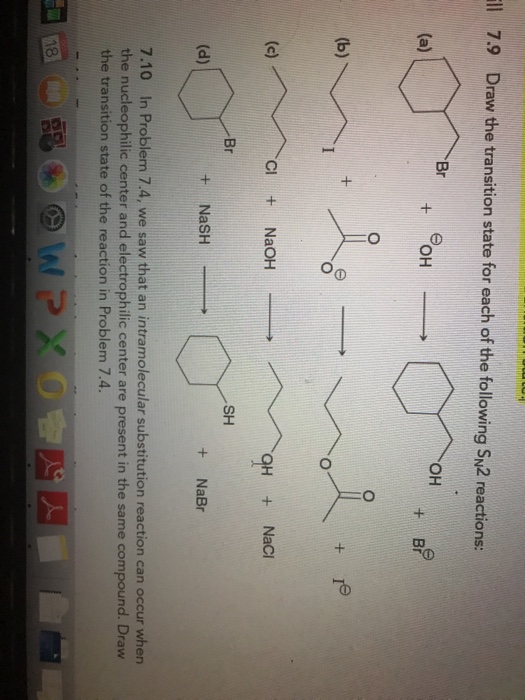
Solved Ll Draw The Transition State For Each Of The Chegg Com
1 Nucleophile back-side attacks the δ carbon center.

. And the question is you have to draw the transition state off the S and foolish. You can put the reactants at any energy level and then draw the. S N 2 is a synchronous mechanism bond formation and bond breaking occur simultaneously in which the attack of.
It would double the rate. Given the following single-step reaction draw the curved-arrow mechanism. Rate of SN2 reaction depending on following different conditions in case of primary halides.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Get both stereoisomers in SN1. Draw the product for each of the following SN2 reactions.
The reaction of 1-bromopropane with sodium iodide gives 1-iodopropane. An SN2 reaction is a concerted bimolecular reaction. In which of the following solvents would the reaction of 1-bromobutane with sodium chloride NaCl proceed the fastest.
Draw the transition state for each of the following SN2 reactions. Which of the following alkyl halides undergoes the fastest SN2 reaction with sodium cyanide NaCN. Draw the structure of reactants and products on the diagram.
The dotted lines on the transition state indicate the bonds that are being formed and broken. Draw the transition state for the following Sn2 reaction. The extent of charge development in a transition state is dependent on the earliness or lateness of that transition state.
Two reacting species are involved in the rate determining step of the reaction. Draw the transition state for the following Sn2 reaction. Draw the transition state for the reaction.
It would reduce by half. Draw the Ukrainian Flag. Draw a reaction coordinate for the following two SN2 reactions.
Draw the carbocation intermediate generated by each of the following substrates in SN1 reaction. Draw the transition state for the reaction between S-1-iodo-3-methylpentane and NaSCH 3. Up to 256 cash back Explain using mechanisms bromomethane and 2-bromo-2-mcthylpropane Show the elimination products and draw the intermediate or transition state for the following reactions.
Explain which reaction mechanism SN2 SN1 E2 or E1 these reaction follow and show the major products. Question 5 The Energy Diagram of SN2 reaction. What is the effect of doubling the concentration of NaI on the rate of the reaction.
In SN2 get only inverted stereoisomer. This type of reaction is also referred to as bimolecular nucleophilic. Up to 256 cash back Draw the transition state for the following SN2 reaction.
Chemistry questions and answers. Which of the following are involved in the transition state and therefore the rate law of SN2 reactions. Substrate alkyl groups Leaving group.
Indicate bonds which are forming and those which are breaking by dashed lines. Consider the following SN2 reaction Assuming no other changes what is the effect on the rate if the concentration of both 1-chloro-3-methylbutane and NaN3 is doubled. Set the route card to the following.
Assume the reaction is exothermic and ΔH -75 kJmol and Ea 50 kJmol. The term SN2 stands for Substitution Nucleophilic Bimolecular. Draw the product of the following reaction by following the curved arrows.
No transition state off this nuclear Felix institution is highly unstable state where the making and breaking off the bond is student This sandwich please. It would triple the rate. Label the axes the Ea the ΔH and the transition state of the reaction.
Learn this topic by watching SN1 SN2 E1. Clearly label the rate limiting step any intermediates generated and the transition state for. For unlimited access to Homework Help a Homework subscription is required.
Draw and name Stereochemistry. P am1 IRCforwardcalcfcmaxpoints300stepsize1recalc10 This means to follow the minimum energy path in the forward see above direction up to 300 points. 2 Transition state forms in which nucleophile is forming bond with carbon while leaving group is breaking its bond.
Substrate alkyl groups Leaving group. NaCN CHs 8 6 NC-------Br NaBr CN CHs 8 ---CHCH H CHỊCH H Br IV a b iv O c 111 d. CH3I NaCN CH3CN.
Label each reaction in the reaction coordinate graph and contrast the two reaction pathways. Concerted means that it involves two reactions taking place at the same time while bimolecular means that the rate determining step involves two molecules. A special Agent circular.
Show all partial charges on appropriate atoms. The cyanide ion attacks the alkyl halide from the rear. Draw and name Stereochemistry.
As the reaction of sodium cyanide with bromomethane follows S N 2 mechanism the highest occupied molecular orbital HOMO of the nucleophile CN - interacts with the anti - bonding orbital LUMO of the leaving group Br -. So in the transition state the C-Br bond is partially broken and at the same time the C-O bond is partially formedNotice that all the bonds are broken and formed in a single step and this is known as a concerted process happening simultaneously. For the following SN2 reaction draw the organic and inorganic products of the reaction and identify the nucleophile substrate and leaving group.
How does this compare to SN2 reactions. Include wedgedash bonds and H on a stereocenter. In the transition state the leaving group Cl- is departing while the nucleophile CN- is forming a bond to the.
3 The leaving group leaves forming the final product. A S-2-Chloropentane and NaSH b R-3-Iodohexane and NaCl c R-2-Bromohexane and sodium hydroxide ANS. 2 marks H CHZBE.
Take the transition state geometry and save it as a new input file MeCl_ts_IRC_forwardgjf and GaussView. The S N 2 reaction is a nucleophilic substitution reaction where a bond is broken and another is formed synchronously. What is the effect of doubling the concentration of NaI on the rate of the reaction.
The value of Δ H for the reaction is 75 k J m o l and the value of Δ S is 54 J K m o l. The reaction of 1-bromopropane with sodium iodide gives 1-iodopropane. Draw an energy diagram for the following S N 2 reaction.
2 Reaction Transition State SN2 summary. Draw the transition state for the reaction. The acetate-hydroxide ion reaction more than one arrow is used in a given mechanism step.
The rate increases by a factor of 2.
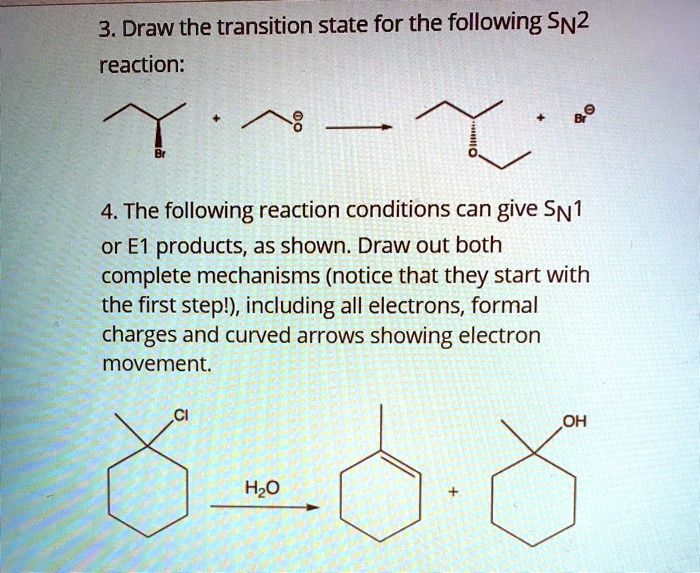
Solved 3 Draw The Transition State For The Following Sn2 Reaction 4 The Following Reaction Conditions Can Give Sni Or E1 Products As Shown Draw Out Both Complete Mechanisms Notice That They Start
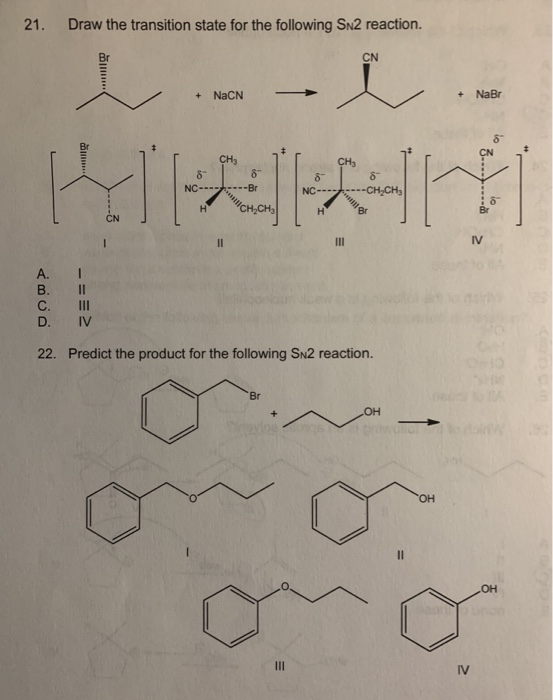
Solved 21 Draw The Transition State For The Following Sn2 Chegg Com
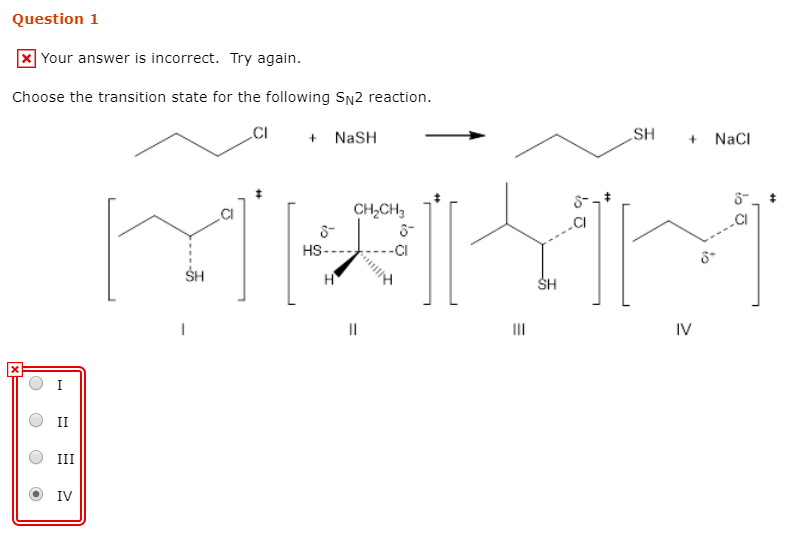
Solved Question 1 Your Answer Is Incorrect Try Again Chegg Com
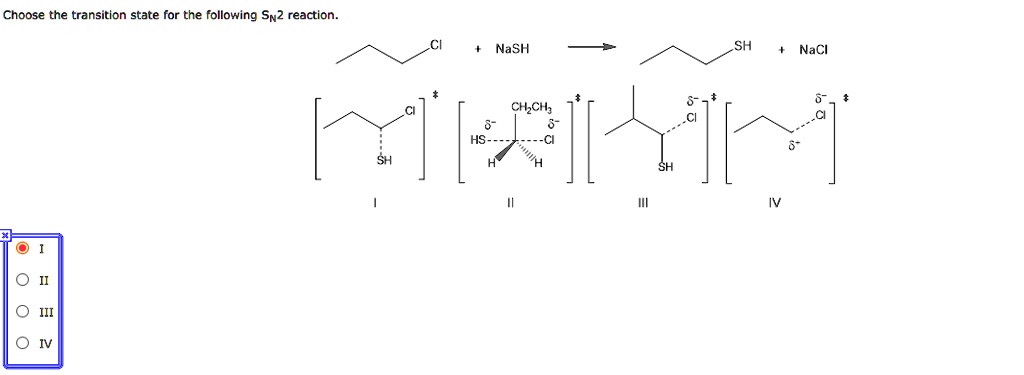
Solved Choose The Transition State For The Following Sn2 Reaction Nash Sh Nacl Chcil Hs
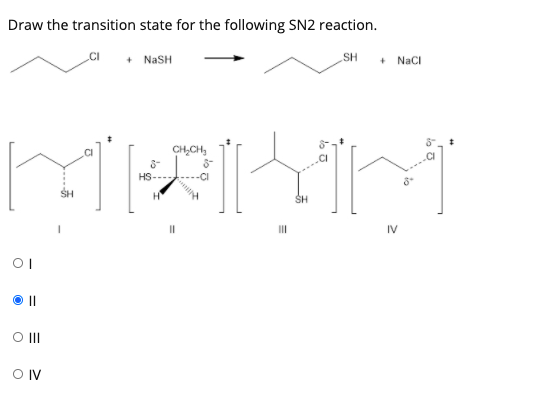
Answered Draw The Transition State For The Bartleby

Oneclass Select The Transition State For The Following Sn2 Reaction Br Cn Ch3 Nc Br Nc Ch2ch3 Ch2

1 Draw The Transition State For The Following Sn2 Reactions Br Sh Nash A Naoh B Homeworklib
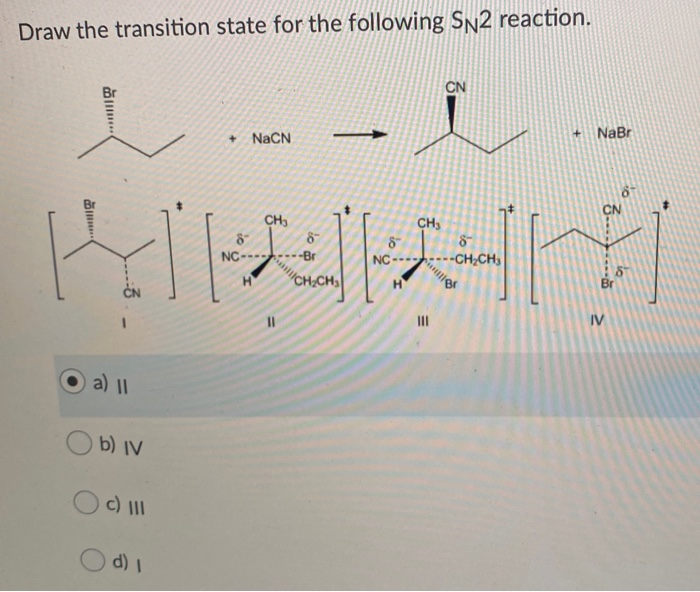
Solved Draw The Transition State For The Following Sn2 Chegg Com
0 comments
Post a Comment